SUMMARY
This is AI generated summarization, which may have errors. For context, always refer to the full article.
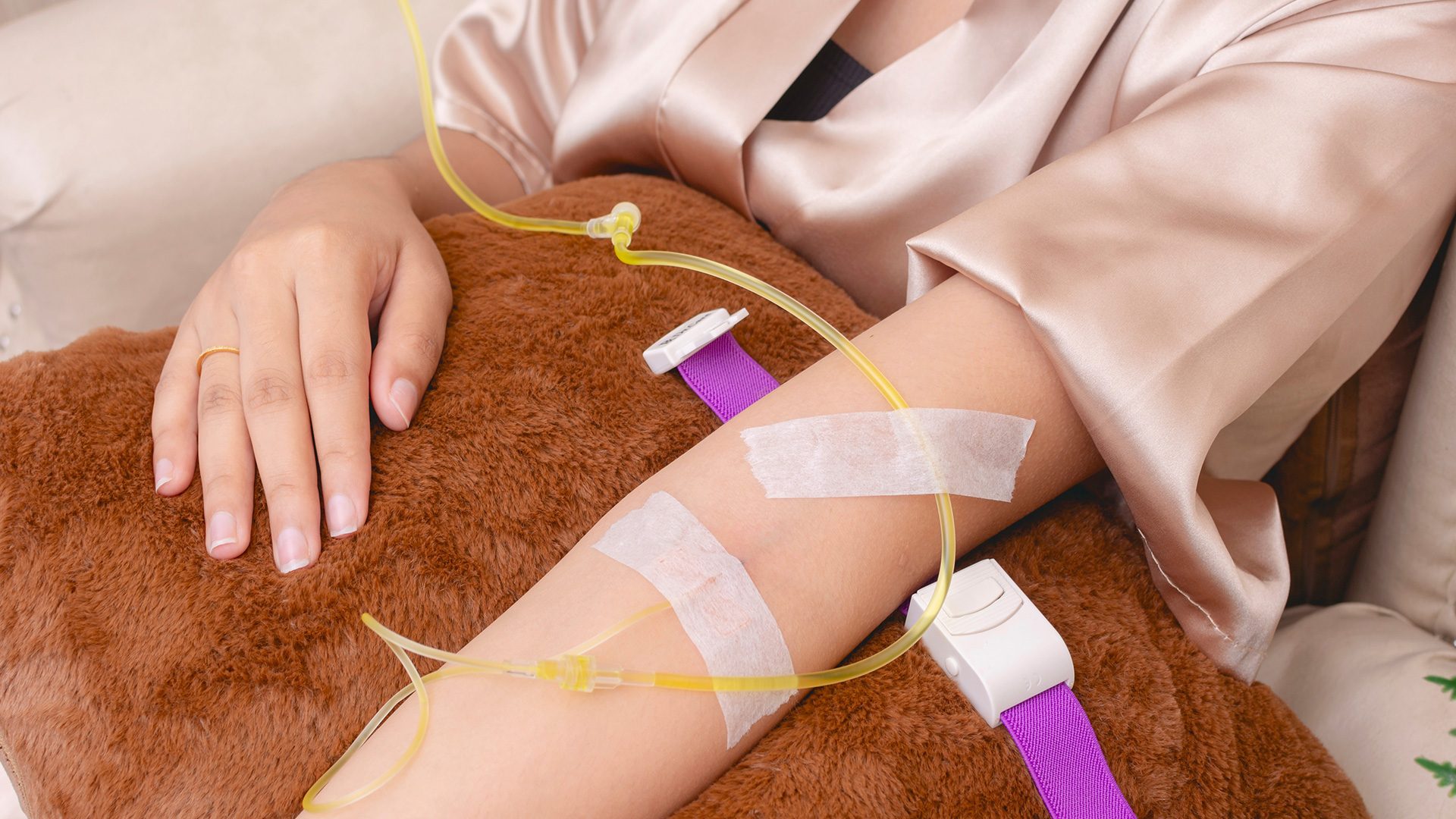
MANILA, Philippines – After photos of Mariel Rodriguez’s glutathione drip session at the Senate made rounds online, Senator Robinhood Padilla shrugged off critics of his wife saying she just “loves to promote good looks and good health.”
But the Philippines has not approved any glutathione products or injectables for cosmetic purposes.
Injectable glutathione, however, is used as a supplementary treatment for patients diagnosed with certain types of cancer, according to the Food and Drug Administration (FDA).
“The Department of Health (DOH) does not support the use of glutathione for skin whitening,” the department said in a statement on Saturday, February 24 – days after Rodriguez’s post went viral.
A 2019 advisory from the FDA also warned the public against using glutathione as a beauty product since there are no available guidelines on its use. The DOH reiterated on Saturday that there are no clinical studies to prove that injectable glutathione can help with skin whitening.
“Avoid buying injectable products online and from being lured to a promising effect of medicines as beauty products,” FDA Advisory No. 2019-182 read.
In a now-deleted Instagram post, Rodriguez noted that the treatment “helps in [so] many ways,” from whitening to affecting one’s immunity.
However, the FDA has cited possible negative side effects that may result from using glutathione, especially when combined with injectable vitamin C, such as possibly getting skin cancer, the formation of kidney stones if the person’s urine is acidic, and hemodialysis for those with glucose-6-phosphate dehydrogenase (G6PD) deficiency.
“Other potential risks include transmission of infectious agents, such as HIV, hepatitis C and B. This is of particular concern when non-medical practitioner administers this treatment or done in a non-sterile facility,” the 2019 FDA advisory read.
Senator Nancy Binay, who chairs the upper chamber’s ethics and privileges committee, raised the alarm on Friday, February 23, since the showbiz personality and her clinic did not notify the Senate that she planned on conducting the IV drip session inside the government building.
The senator also noted that it was done “without the proper medical advice from a licensed health professional.”
Senator Nancy Binay on gluta drip session of Mariel Rodriguez at the Senate.
— Bonz Magsambol (@bnzmagsambol) February 23, 2024
“As public figures, sana aware din tayo sa responsabilidad natin sa publiko… Isipin din natin may kasamang kapanagutan ang pagiging artista, lalo na kung senador ang asawa mo.” @rapplerdotcom pic.twitter.com/RM3bvG05Or
Rappler has reached out to Rodriguez’s IV drip provider – Luxe in Drip PH – via phone call on Saturday morning, but they said the management will reach out soon. We will update this story once they do.
IV drips have gained popularity in the Philippines, with several clinics offering a variety of glutathione IV drip procedures. The DOH called on those who experience negative side effects from injectables, including those involving glutathione procedures, to report their experience to the FDA and seek legal help on top of medical attention, should it be needed. – with reports from Bonz Magsambol/Rappler.com
Add a comment
How does this make you feel?

![[PANOORIN] Naku! Mag-ingat sa unregistered health products online!](https://www.rappler.com/tachyon/2023/05/fact-check-ls-3.jpg?resize=257%2C257&crop=283px%2C0px%2C720px%2C720px)


There are no comments yet. Add your comment to start the conversation.